Multiplex family study data in autism spectrum disorder help us uncover novel ASD-risk genes that primarily display contribution from rare inherited mutations.
Elizabeth Ruzzo and her colleagues tried to identify both rare de novo and inherited genetic risk factors in ASD, they surveyed the whole genome sequences of 2308 individuals from 493 multiplex ASD families i.e. families possessing more than one affected children.
ASD is a neurodevelopmental disorder, individuals with ASD display early obligations in social communication and interaction coupled with bounded and redundant patterns of behaviour, activity or interest (American Psychiatric Association, 2013).
Are high-risk inherited variants only transmitted to affected children and whether genes harbouring them are inter-connected?
The researchers were successful in identifying 98 unique genes that harboured high-risk inherited variants. These high-risk genes were only transferred to affected children and not the unaffected ones.
They also observed that the protein products of these 98 unique genes form substantial direct and indirect interaction network, further suggesting the function of these genes in ASD biology.
NR3C2: the gene of interest
Out of the 98 unique genes, the team directed their attention on NR3C2. NR3C2 is of particular interest because it has not been consistently linked with ASD before (De Rubeis et al., 2014; Sanders et al., 2015). Thus, the team provided the first proof of inherited risk in NR3C2.
They were capable of specifying a new syndromic form of ASD which was distinguished by metacarpal hypoplasia, a high arched palate, sensory hypersensitivity and abnormal prosody.
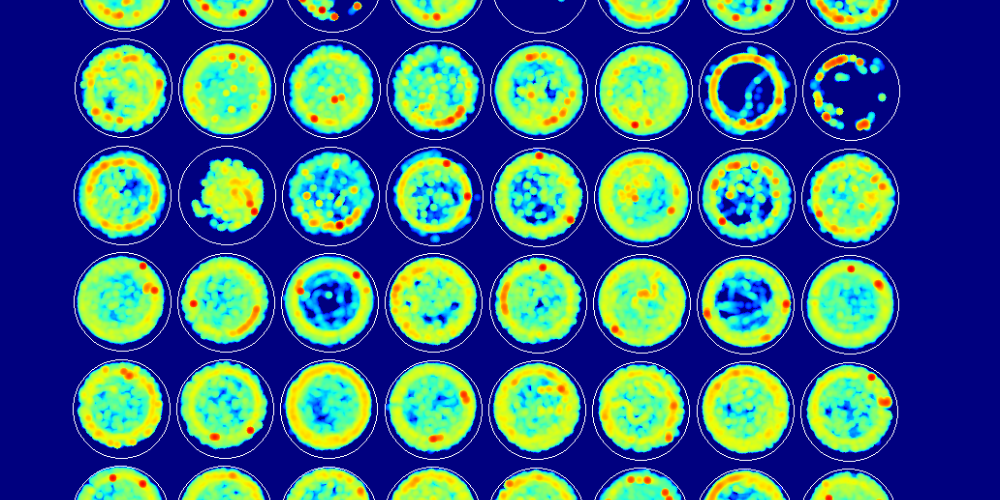
ZebraBox and VideoTracking system: The perfect pair for automated surveillance
The investigators created NR3C2 mutant zebrafish using CRISPR/Cas9. Social interaction and quality of sleep were the two parameters selected for the investigation.
In social preference assay, zebrafish were placed in a flat-bottom 12-well plate. This plate was then positioned on a custom made ZebraBox that was elucidated with infrared and LEDs. ZebraBox enabled the investigators to gain total control over experimental conditions.
To survey locomotor activity the investigators employed an automated VideoTracking system with a Dinion one-third inch monochrome camera equipped with a fixed-angle megapixel lens and infrared filter.

Employing ZebraBox and automated VideoTracking system investigators reported that compared to their wild-type siblings, mutant zebrafish didn't show a social preference to their own kind.
Similarly, ZebraBox and automated VideoTracking system were used for experimenting sleep-wake behaviours in zebrafish. The investigators discovered that mutant zebrafish were more active and slept less compared to their wild-type siblings, mimicking the behaviour observed in individuals with ASD.
Thus the investigators successfully demonstrated that mutants have social behavioural deficits and sleep disturbances, and eventually established NR3C2 as an ASD risk candidate.
TADA test reveals 16 novel ASD risk genes enriched for inherited variation
To further endow the study, scientists employed TADA (transmitted and de novo association) test for identifying ASD risk genes combining both de novo and inherited signals.
TADA mega-analysis identified 69 genes that were significantly correlated with ASD. 16 out of these 69 genes were novel ones and compared to de novo they had an increased amount of risk variants from inherited factors.
Using gene set enrichment analysis, the scientists found these 69 genes to be enriched in a highly co-expressed group of transcriptionally co-regulated genes.
Earlier research has shown how pathways involving largely de novo variation are monopolized by transcriptional and chromatin regulation (De Rubeis et al., 2014). Therefore, the scientists in this study employed gene ontology enrichment analysis to study the same in inherited ASD risk variants. They discovered unique biological pathways that involved cell cycle, ion transport and microtubule cytoskeleton.
After gaining biological insights from known and novel ASD genes, the researchers tried to understand whether these 69 risk genes interact with the 98 unique genes that harboured high-risk inherited variants.
Ruzzo and her team reported a significant protein-protein network established by these 165 genes further confirming the involvement of 98 unique genes in ASD.
The road ahead
To date, research has revealed several de novo variants linked to ASD. In this study, Ruzzo and her team have identified new genes that hugely exhibit contribution from rare inherited mutations.
They believe with an increase in sample size it is possible to unearth several new genes associated with ASD. Furthermore, it will help in exploring the additive effects of both common and rare in-inherited variation.
The team is confident that the pattern of interaction they have postulated between ASD risk genes and their interconnected-ness at the gene level will be exploited further in future studies.
References
-
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub.
-
De Rubeis, S., He, X., Goldberg, A. P., Poultney, C. S., Samocha, K., … Fromer, M. (2014). Synaptic, transcriptional and chromatin genes disrupted in autism. Nature, 515(7526), 209–215.
-
Ruzzo, E. K., Pérez-Cano, L., Jung, J.-Y., Wang, L., Kashef-Haghighi, D., Hartl, C., … Wall, D. P. (2019). Inherited and De Novo Genetic Risk for Autism Impacts Shared Networks. Cell, 178(4), 850–866.e26 .
-
Sanders, S. J., He, X., Willsey, A. J., Ercan-Sencicek, A. G., Samocha, K. E., Cicek, A. E., … State, M. W. (2015). Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron, 87(6), 1215–1233 .