For the first time ever, researchers demonstrate a strong relationship between sociability, development, neuronal loss and motor performances in a mouse model of autism spectrum disorder (ASD). In this study, pregnant female mice received valproic acid (VPA) at embryonic day 12.5. Later, the offspring underwent a series of behavioral analyses before being slain for histological analogy in the nigrostriatal pathway, motor cortex and cerebellum.
Autism spectrum disorder and motor impairments
Autism is one of the most common neurological disorders seen in children. Currently, there are no standardized diagnostics or treatments available for autism. Data suggest that males are more prone to autism than females, with a constantly increasing prevalence to attain a ratio of 1 in 68 individuals Current DSM-5 classification recognizes 2 global symptoms as hallmarks of ASD: (i) disorders of social communication and (ii) restricted and repetitive behavior. Initial reports on autistic patients reveal early motor deficits with clumsy gait. Since then, motor impairments have been receiving increased attention in ASD research studies. For example, there are a growing number of studies mentioning impairments in limb coordination, visio-motor and manual dexterity tasks.
Developmental milestones and core ASD symptoms of VPA mice
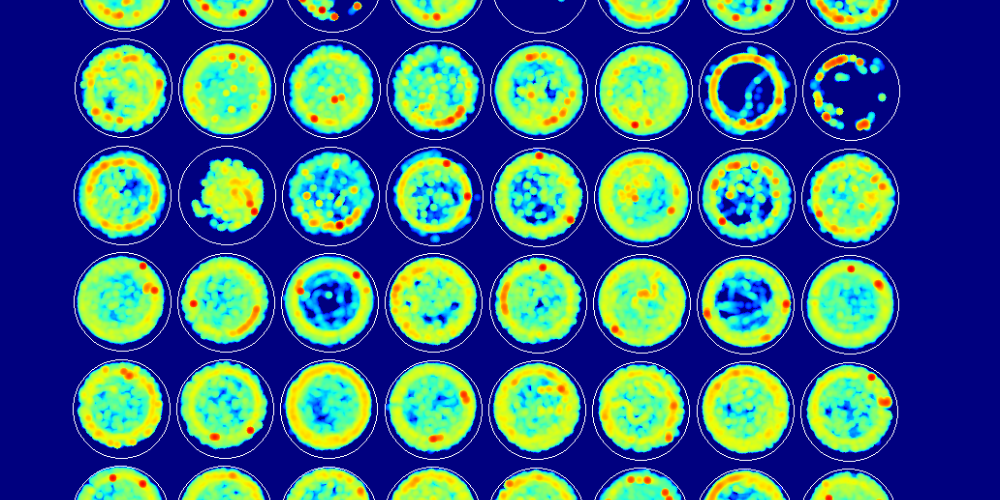
To assess the developmental milestones, experimenters studied early postnatal life (1-3 weeks) of pups for righting reflex and eye-opening. They observed that VPA male mice showed significant developmental delays, which were more pronounced in VPA males than VPA females. Post-hoc analysis of social behavior in 5-6 week-old mice showed an astonishing 7-fold decrease in the sociability of VPA male mice. Though regardless of the treatment, female mice demonstrated similar sociability index.
Assessment of motor coordination using primary SHIRPA screen and challenging beam
The primary SHIRPA screen was used for identifying global disturbances in posture, gait and muscle tone as well as coordination and motor control abnormalities. VPA mice showed altered behavior in the SHIRPA screen test, where only VPA males demonstrated a 3-fold increase in immobility along with a significant decrease in locomotor activity. On the challenging beam test, the time taken by VPA mice to transverse the beam was not affected, but VPA exposure significantly increased the errors per step in both males and females.
GaitLab assesses temporal, spatial and kinetic gait parameters
Gait of VPA mice were analyzed using GaitLab, an automated gait analysis system. The apparatus consists of a 1.5 m long glass corridor, dimly lit with a green light.
This green light is beamed into the glass walkway, with the light reflecting downward and a high-definition camera capturing the spatial and kinetic parameters of the footprints. Stride length, limb base of support and pair gap were the three parameters analyzed with GaitLab. Researchers observed that VPA exposure did not affect the paw area, regularity or speed, but it significantly affected the pair gap in both males and females. GaitLab can provide several information the locomotion of the subjects: With the limb base of support, the post-hoc analysis demonstrated a significant increase in both the right and left limb base of support for VPA males and females, indicating the presence of a significant ataxic gait. Similar data were observed with stride length, as both VPA males and females showed a decrease in their stride length. Altogether, GaitLab significantly helped the researchers to detect major deficits in gait after VPA exposure in mice.
VPA exposure induces cell loss in motor brain areas
Post behavioral analysis, the researchers focused their attention on tissue processing and immunohistochemistry. Males and females were randomly selected for histopathological analysis. Purkinje cells (PC) in the cerebellum, striatum and motor cortex neurons and dopaminergic neurons of the substantia nigra were quantified to study the cell loss. In the cerebellum, lobules VI and VII are found to be most affected in ASD. (5,6) On studying these two lobules, the researchers found no effect of VPA exposure in the PC of lobule VI whereas Lobule VII showed a sex-dependant and specific regional loss of PC.
Further to understand the relationship between motor behavior and loss of cortical neurons, the researchers quantified NeuN immunoreactivity neurons in both the primary and secondary motor cortex. The post-hoc analysis showed substantial cortical cell loss only in males and not females, indicating sex-dependant alteration due to VPA treatment in the motor cortex. Lastly, after studying the VPA exposure on the nigrostriatal pathway, researchers saw no loss of striatal or dopaminergic neurons, suggesting that the deficits in motor functions cannot be credited to the loss of nigral or striatal neurons.
Is there a relationship between histopathological and behavioral findings?
Correlation analysis was performed in order to determine any potential links between histopathological and behavioral data. Interestingly males had more correlations than females. Collectively, four major links were revealed in males.
1. Early developmental delays correlated with motor impairments that were assessed later, along with deficits in social interaction and neuronal loss in the motor cortex and Crus I.
2. Motor impairments correlated with neuronal loss and deficits in social interaction.
3. Correlation between neuronal loss and decrease in sociability index.
4. Inter-relatedness of motor impairments, neuronal loss, developmental delays and sociability in males.
Conclusion
This study suggests that motor impairments could possibly contribute to communication and social deficits in ASD and these deficits may share mutual neuronal substrates in the cerebellum. Thereby, the investigators demonstrate the relevancy and ease of implementing motor impairments as a biomarker of ASD. They suggest that the implementation of biomechanical assessment of motor abilities in children can be a precious tool in the diagnosis of ASD. The study also suggests the use of motor behavior and histopathological data in the development of therapeutics that focuses on specific motor brain areas in the future.
References
1. CDC (2014) Health, United States, 2014: with special feature on adults aged 55–64. Hyattsville, MD: National Center for Health Statistics.
2. Loomes R, Hull L, Mandy WPL (2017) What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 56:466–474.
3. APA (2013) Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Publishing.
4. Fatemi SH, et al (2012) Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11:777–807.
5. Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, Schreibman L (1994) Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR Am J Roentgenol 162:123–130.
6. Skefos J, Cummings C, Enzer K, Holiday J, Weed K, Levy E, Yuce T, Kemper T, Bauman M (2014) Regional alterations in purkinje cell density in patients with autism. Plos One 9:e81255.
7. Al Sagheer, T., Haida, O., Balbous, A., Francheteau, M., Matas, E., Fernagut, P. O., & Jaber, M. (2018). Motor impairments correlate with social deficits and restricted neuronal loss in an environmental model of autism. International Journal of Neuropsychopharmacology, 21(9), 871-882.
Related products
VideoTrack, PhenoRack, and Sleep Deprivatio